15+ Calculate The Energy Of The Photon Emitted For Transition A
Unlike in classical mechanics quantum systems constantly fluctuate in their lowest energy state as described by the Heisenberg uncertainty principle. Web The sidewall emission increases the ratio of light emitted to large angles 70 leading to a larger F which lowers the ratio of light contributing to the on-axis intensity.
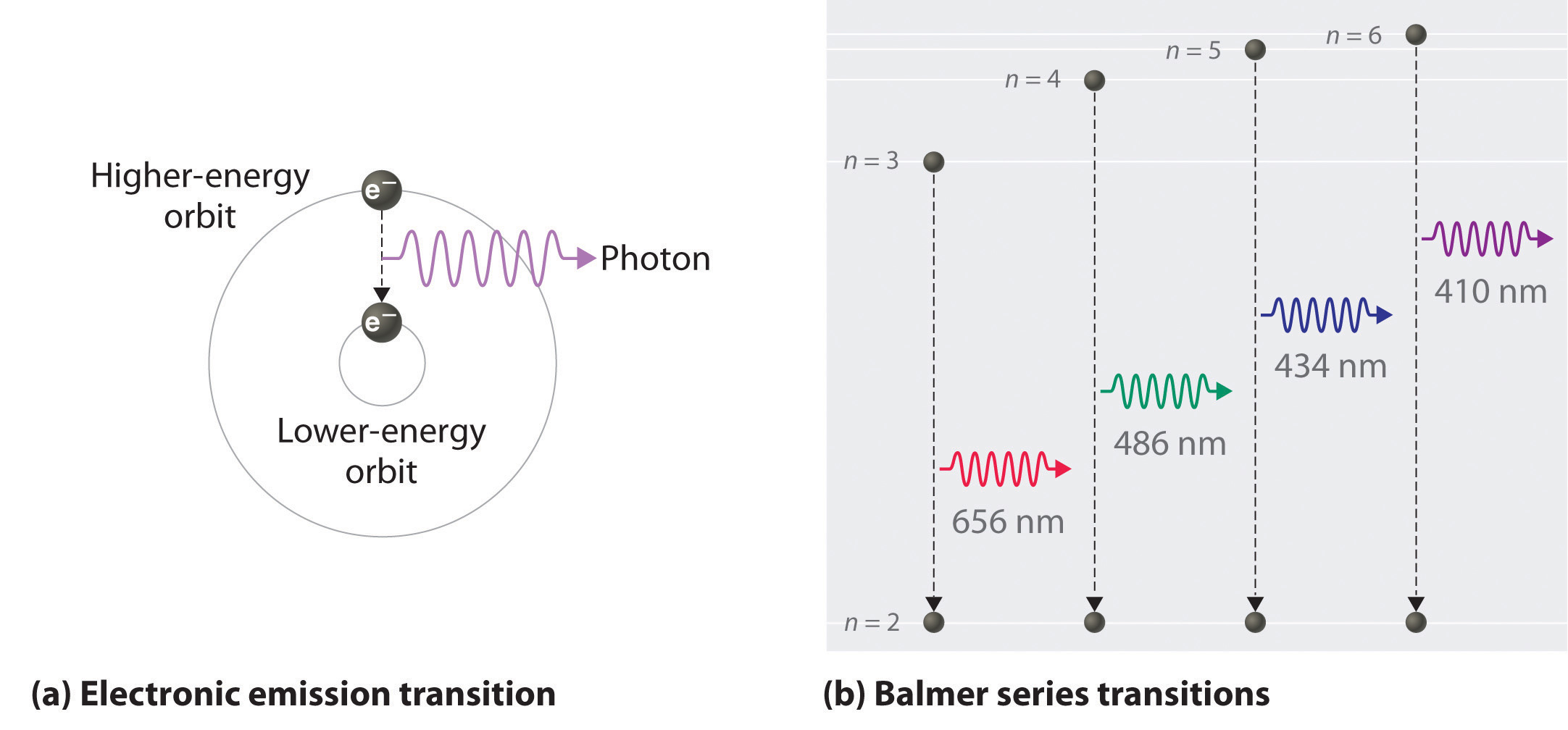
How Much Energy Is Released When 1 Mol Of Hydrogen Atoms Transition From N 3 To N 2 Socratic
Web Microsoft pleaded for its deal on the day of the Phase 2 decision last month but now the gloves are well and truly off.
. Web This photon can take on a large range of frequencies from ultraviolet radiation to visible light to infrared waves and the frequency of the emitted photon is wholly dependent upon which energy. The main gamma-ray of Barium-137m is 661keV photon. It is a form of inelastic light scattering where a photon excites the sample.
22 February 1857 1 January 1894 was a German physicist who first conclusively proved the existence of the electromagnetic waves predicted by James Clerk Maxwells equations of electromagnetismThe unit of frequency cycle per second was named the hertz in his. Web The Planck constant or Plancks constant is a fundamental physical constant of foundational importance in quantum mechanicsThe constant gives the relationship between the energy of a photon and its frequency and by the mass-energy equivalence the relationship between mass and frequencySpecifically a photons energy is equal to its. 671 million miles per hour.
To fully validate general relativity it is important to also show that the rate of arrival of the photons is greater than the rate at which they are emitted. Recall that the. 6 to 30 characters long.
Inelastic scattering means that the energy of the emitted photon is of either. The two modes for each set of n i correspond to the two polarization states of the photon which has a spin of 1. Web After you obtain the energy then you can realize that that energy has to correspond exactly to the energy of the photon that came in.
Electronic transition from the n 1 to the n 4 principal energy level. This excitation puts the molecule into a virtual energy state for a short time before the photon is emitted. Web Gamma rays are emitted by unstable nuclei in their transition from a high-energy state to a lower state known as gamma decay.
In the following we will. According to the special theory of relativity c is the. The energy and momentum of a photon depend only on its frequency or inversely its wavelength λ.
Calculate the primary photon dose rate in sieverts per hour Svh-1 at the outer surface of a 5. Calculate the frequency of the light emitted by a hydrogen atom during a transition of its. This effect is more.
Therefore even at absolute zero atoms and molecules retain some vibrational motionApart from atoms. Colorblueulcolorblacknu lamda c Here. This vacuum energy of the electromagnetic field is responsible for the Casimir effect.
Thus the wavelength is. Web Nature of Decay Barium-137m is a product of a common fission product Caesium 137. In most practical laboratory sources the excited nuclear states are created in the decay of a parent radionuclide.
Web In empty space the photon moves at c the speed of light and its energy and momentum are related by E p c where p is the magnitude of the momentum vector pThis derives from the following relativistic relation with m 0. Nu is the frequency. Web X-ray fluorescence XRF is the emission of characteristic secondary or fluorescent X-rays from a material that has been excited by being bombarded with high-energy X-rays or gamma raysThe phenomenon is widely used for elemental analysis and chemical analysis particularly in the investigation of metals glass ceramics and building materials and for.
186000 miles per second. Where k is. Web How to Find the Frequency of a Photon Emitted by an Electron Transition.
Read the problem and locate the values for the quantum number of the lower state eqrmn_low. Microsoft describes the CMAs concerns as misplaced and says that. Web In other words the higher energy of the photon after it falls can be equivalently ascribed to the slower running of clocks deeper in the gravitational potential well.
Web The photon energy necessary to eject electrons from the metal is called the theshold energy - at the threshold frequency. Following the inflationary period the universe continued. When the atom is in a magnetic field FLPB however there are many more lines.
Web The water-splitting reaction using photocatalyst particles is a promising route for solar fuel production 1234Photo-induced charge transfer from a photocatalyst to catalytic surface sites is. Web Zero-point energy ZPE is the lowest possible energy that a quantum mechanical system may have. For r 0 the energy of the mode is not zero.
Must contain at least 4 different symbols. As was written atomic nuclei consist of protons and neutrons which attract each other through nuclear forceIn contrast protons repel each other via electromagnetic force due to their positive charge. Web The magnitude of the Raman effect correlates with polarizability of the electrons in a molecule.
Web Heinrich Rudolf Hertz h ɜːr t s HURTS. DeltaE E_photon hnu hclambda where h is Plancks constant c is the speed of light and lambda is the wavelength of the incoming photon. Web In physics the radiative efficiency limit also known as the detailed balance limit ShockleyQueisser limit Shockley Queisser Efficiency Limit or SQ Limit is the maximum theoretical efficiency of a solar cell using a single p-n junction to collect power from the cell where the only loss mechanism is radiative recombination in the solar cell.
Web Now in order to find the energy that corresponds to this transition calculate the frequency nu of a photon that is emitted when this transition takes place by using the fact that wavelength and frequency have an inverse relationship described by this equation. ASCII characters only characters found on a standard US keyboard. Web In physical cosmology cosmic inflation cosmological inflation or just inflation is a theory of exponential expansion of space in the early universeThe inflationary epoch lasted from 10 36 seconds after the conjectured Big Bang singularity to some time between 10 33 and 10 32 seconds after the singularity.
Identify the principal quantum number of the initial energy state eqn_i eq and the principal quantum number. Web The speed of light in vacuum commonly denoted c is a universal physical constant that is important in many areas of physicsThe speed of light c is exactly equal to 299792458 metres per second approximately 300000 kilometres per second. Web How to Calculate the Photon Frequency Absorbed or Emitted by an Electron in a Hydrogen Atom.
A very accurate. Web 1 The quantum number r can be interpreted as the number of photons in the mode. Web The transitions between state ketslslIV and any one of the others occurs with the absorption or emission of a photon whose frequency 1420 megacycles is 1h times the energy difference 4A.
Web where E is the photon energy E EA is the electron affinity and l is the angular momentum quantum number of the outgoing electron 30In the case of oxygen the 2p electron can be emitted as an s.
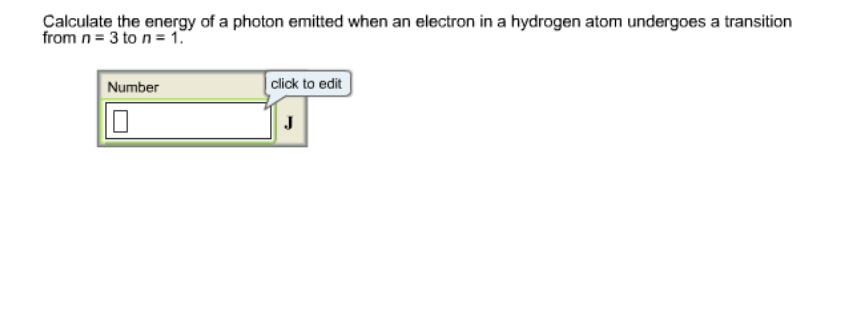
Solved Calculate The Energy Of A Photon Emitted When An Chegg Com

The Photon Emitted Due To Electronic Transition From 5 Th Excited State To 2 Nd Excited State In Li 2 Is Used To Excite He Ion After Absorbing The Photon Electron Reaches In

Coordination Directed Self Assembly Of Functional Polynuclear Lanthanide Supramolecular Architectures Chemical Reviews

Quantum Theory Of The Atom Ppt Download

Nuclear Fusion Building Today The Energy Of Tomorrow By Esteyco Sap Issuu
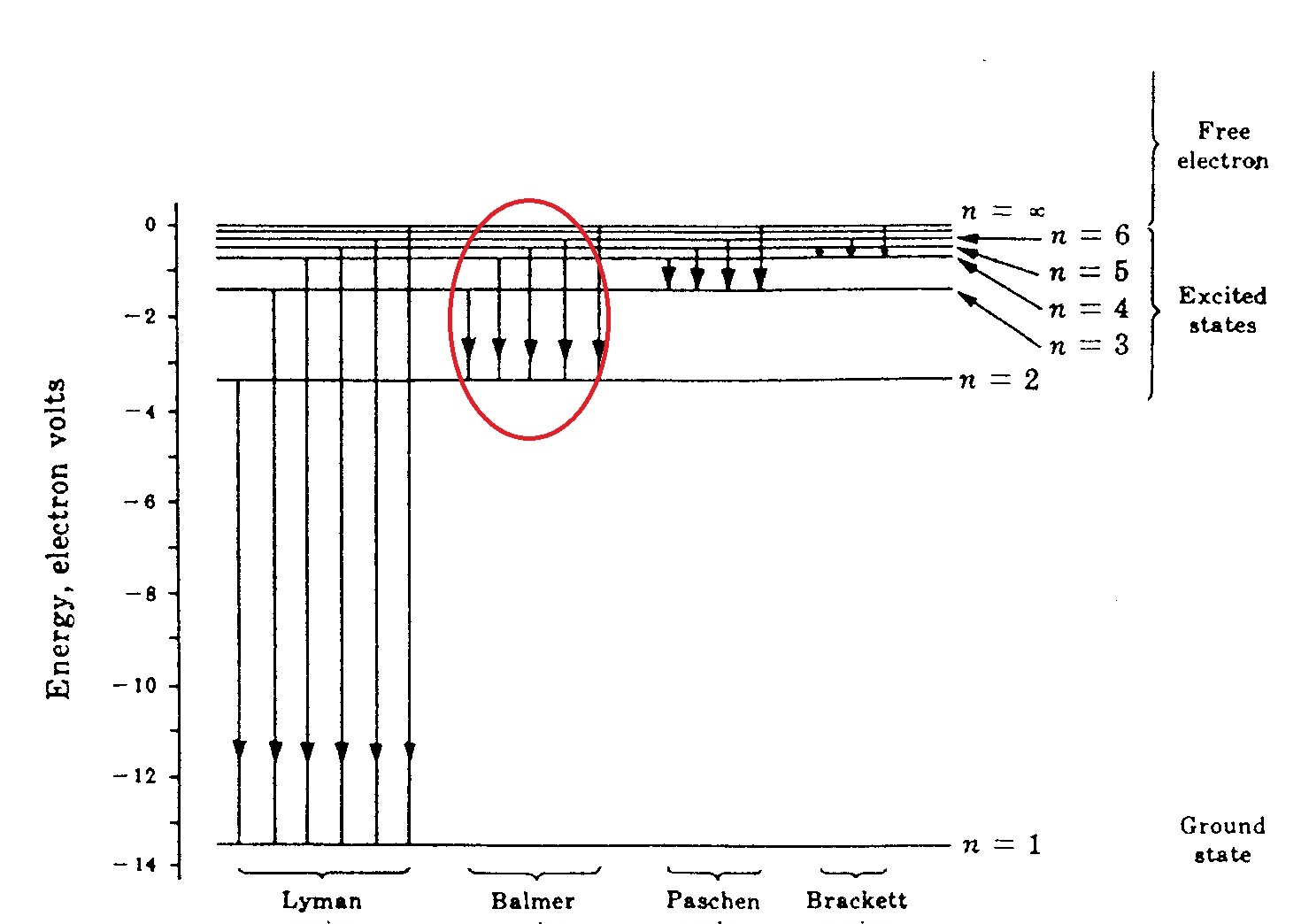
What Is The Wavelength In Nm Of A Photon Emitted During A Transition From The N 5 State To The N 2 State In The Hydrogen Atom Socratic
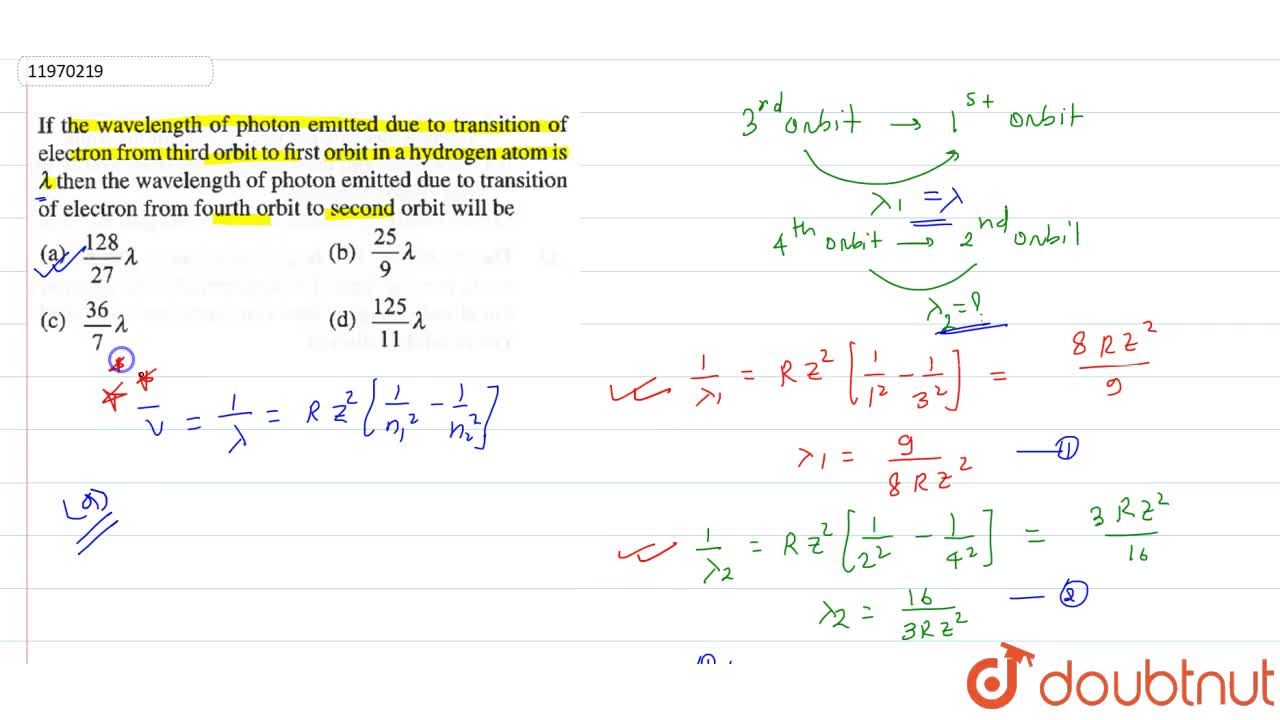
If The Wavelength Of Photon Emitted Due To Transition Of Electron From Third Orbit To First Orbit In A Hydrogen Atom Is Lambda Then The Wavelength Of Photon Emitted Due To Transition
Full Article Hydrogenic Systems Frequency Standards And Fundamental Constants

The Energy Of Photon Emitted Corresponding To Transition N 3 To N 1 Is H 6 10 34 J Sec

Calculate The Energy Frequency Wavelength Of An Electron Transition In The Bohr Atom Youtube

Electron And Nuclear Dynamics In Many Electron Atoms Molecules And Chlorophyll Protein Complexes A Review Sciencedirect

Auroral X Ray Emission At Jupiter Depth Effects Ozak 2010 Journal Of Geophysical Research Space Physics Wiley Online Library

The Energy Of Photon Emitted Corresponding To Transition N 3 To N 1 Is H 6 10 34 J Sec

Auroral X Ray Emission At Jupiter Depth Effects Ozak 2010 Journal Of Geophysical Research Space Physics Wiley Online Library

High Energy Density Physics With Intense Ion And Laser Beams Gsi

Full Article Exclusion Principle For Photons Spin Statistic Selection Rules For Multiphoton Transitions In Atomic Systems
Soft X Ray Spectral Analysis Of Laser Produced Molybdenum Plasmas Using The Fundamental And Second Harmonics Of A Nd Yag Laser